Top AI in Pharma & Healthcare in 2022
December 1, 2022Physical Requirements of Artificial Intelligence
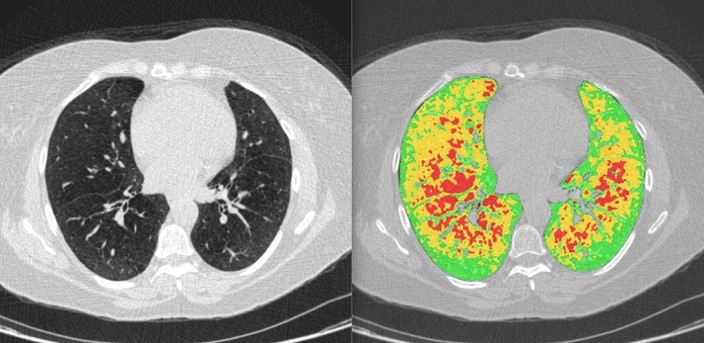
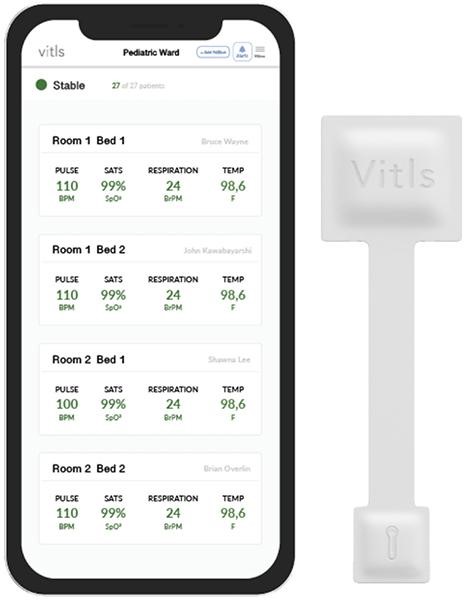
December 1, 2022FDA Cleared Imbio an AI programs for scanning lung diseases; Neuromuscular Tongue Stimulator for Snoring & Vitls Platform for Remote Patient Monitoring
AI programs have been developed to scan lung disease, treat OSA, and perform remote patient monitoring...